

Thomson was also able to measure the ratio of the charge of the electron to its mass, q e q e / m e / m e -an important step to finding the actual values of both q e q e and m e m e. Additionally, he collected the rays in a metal cup and found an excess of negative charge.

(See Figure 30.5 and Figure 30.6.) He verified the negative charge of the cathode rays with both magnetic and electric fields.
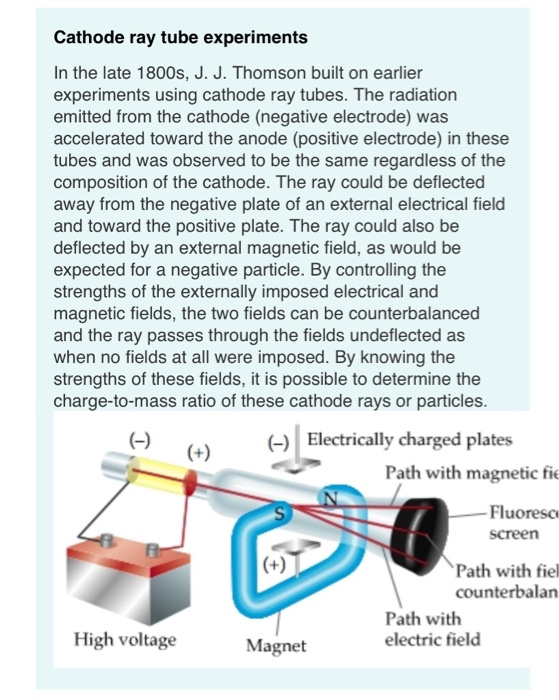
Thomson (1856–1940) improved and expanded the scope of experiments with gas discharge tubes. When a magnetic field is applied, the beam bends in the direction expected for negative charge. Once called Geissler tubes and later Crookes tubes, they are now known as cathode-ray tubes (CRTs) and are found in older TVs, computer screens, and x-ray machines. Electrons emitted from the cathode are accelerated toward the anode they excite atoms and molecules in the gas, which glow in response. These were the first direct indications of electrons and their charge.įigure 30.4 A gas discharge tube glows when a high voltage is applied to it. He also found that their normally straight path is bent by a magnet in the direction expected for a negative charge moving away from the cathode. Crookes showed that the electrons carry momentum (they can make a small paddle wheel rotate). Gas discharge tubes today are most commonly called cathode-ray tubes, because the rays originate at the cathode. These “ cathode rays” collide with the gas atoms and molecules and excite them, resulting in the emission of electromagnetic (EM) radiation that makes the electrons’ path visible as a ray that spreads and fades as it moves away from the cathode. The English scientist William Crookes, among others, continued to study what for some time were called Crookes tubes, wherein electrons are freed from atoms and molecules in the rarefied gas inside the tube and are accelerated from the cathode (negative) to the anode (positive) by the high potential. They were first studied seriously by Heinrich Geissler, a German inventor and glassblower, starting in the 1860s. These tubes were the precursors to today’s neon lights. When a high voltage is applied to the electrodes, the gas glows. Gas discharge tubes, such as that shown in Figure 30.4, consist of an evacuated glass tube containing two metal electrodes and a rarefied gas. We will now explore the discovery of the electron and nucleus as substructures of the atom and examine their contributions to the properties of atoms. We have also covered many aspects of the electric and magnetic forces that affect charges. In previous discussions, we have noted that positive charge is associated with nuclei and negative charge with electrons.


 0 kommentar(er)
0 kommentar(er)
